November 28, 2017
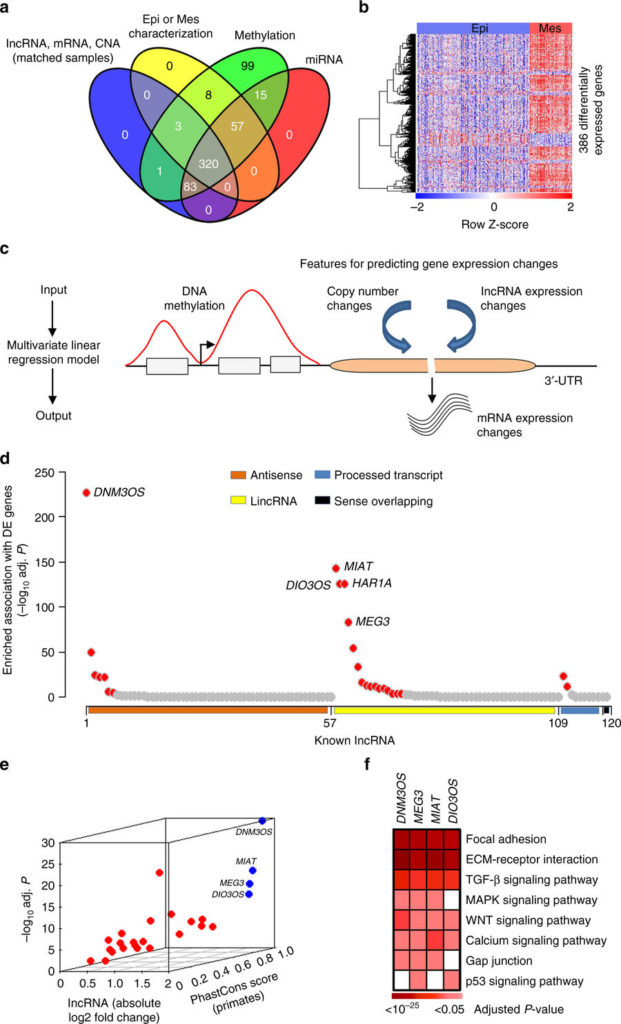
Identifying critical lncRNA in ovarian cancer EMT. a Ovarian cancer patients (n = 320) with genomic and molecular profiling data that classified into epithelial (Epi; n = 231) or mesenchymal (Mes; n = 89) subtypes were selected for analysis. b Heatmap of 386 genes that were differentially expressed in the mesenchymal subtype compared with the epithelial subtype. cInferring deregulatory programs from ovarian cancer profiling data. Change in mRNA expression is modeled as linear function of the gene’s DNA methylation, copy number, and lncRNA expression. d, e Systematic prediction of EMT-linked lncRNA from the lncRNA-gene association information obtained from the linear model. d The lncRNA that had significantly enriched association with the differentially expressed genes (n = 25, red dots; top 5 lncRNA labeled) were inferred as EMT associated. Remaining lncRNA were represented by gray dots. The X-axis with four different colors represent major annotation classes of the selected lncRNA (n = 120). The Y-axis denotes which lncRNA had enriched association with the differentially expressed genes compared with non-differentially expressed genes. e Filtering of high confidence EMT-linked lncRNA (n = 4; blue dots with labels) based on their aberrant expression (X and Y-axis) in EMT and conservation score (Z-axis). Gray dots represent remaining lncRNA. f Heatmap shows significantly enriched association of the inferred lncRNA with EMT-linked pathways. For d and f, P-values determined by BH adjusted hypergeometric test
Nature Communications
ISSN 2041-1723 (online)
Ovarian cancer is the most lethal gynecologic malignancy in the United States, resulting in an estimated 14,100 deaths and 22,500 new cases in 2017 alone. This high mortality is primarily caused by resistance to therapy and the diagnosis of ovarian cancer after it has already metastasized, which occurs in approximately 80 percent of patients.
A new study from Sidney Kimmel Cancer Center (SKCC) at Thomas Jefferson University investigator Christine Eischen, PhD, provides new insights into the mechanisms contributing to ovarian cancer. The Eischen group focused on the role of long non-coding RNAs (lncRNAs), which have emerged as key regulators of genes. By evaluating the molecular changes that occur in large cohorts of ovarian cancer patients, the researchers were able to identify several lncRNAs that are linked to the disease. These lncRNAs were reproducibly altered in patients, and are responsible for a shift in cellular function that contributes to the metastatic properties of the cancer cells.
The research, which appears in a recent issue of Nature Communications, was spearheaded by lead author and bioinformatician Dr. Ramkrishna Mitra, a postdoctoral associate in the Eischen laboratory. Dr. Mitra undertook a large-scale bioinformatics approach to evaluate over 700 ovarian cancer molecular profiles from four patient cohorts. This analysis led to the identification of several lncRNAs that are overexpressed in a particular subset of ovarian cancer, those that are thought to be the most aggressive.
Further analysis revealed that overexpression of these lncRNAs in turn changed the expression of proteins that regulate a well-known developmental process, termed the epithelial-to-mesenchymal transition (EMT). EMT is important for cell migration and invasion — two characteristic of metastatic cancer cells — strongly suggesting that the link between lncRNAs and EMT contributes to the metastatic progression of ovarian cancer.
Following up on this idea, the researchers found that one of the lncRNAs was directly implicated in patient outcomes. “Overexpression of one of the lncRNAs, DNM30S, was significantly correlated with worse overall ovarian cancer patient survival,” said Dr. Eischen.
Based on these observations, the researchers suggest that targeting of the lncRNAs might represent a viable treatment strategy for ovarian cancer. To test this idea, they experimentally reduced the expression of the DNM30S lncRNA, which resulted in reduced ovarian cancer cell migration and invasion. In future work, the Eischen laboratory aims to further understand the role of lncRNAs in ovarian cancer, and potentially translate their findings into clinical applications to reduce ovarian cancer metastasis.
Ramkrishna Mitra, Xi Chen, Evan J. Greenawalt, Ujjwal Maulik, Wei Jiang, Zhongming Zhao, Christine M. Eischen. Decoding critical long non-coding RNA in ovarian cancer epithelial-to-mesenchymal transition. Nature Communications, 2017; 8 (1) DOI: 10.1038/s41467-017-01781-0